Food and drugs safety agencies have a hard enough time overseeing the safety of properly regulated products. Imagine the mess with cannabis products.
“We base decisions on product labels and Certificates of Analysis – for example, we know how many calories are in a McDonald’s Big Mac or the salt content in potato chips – and choose whether or not to eat those items based on knowledge gained from the labels. Consumers in the cannabis space are not afforded this same degree of confidence.”
These are the words of Dr. Susan Audino, closely and actively involved in the standardization of laboratory testing in the cannabis industry, and one of the very few people in the space who is truly independent.
She was also one of the presenters at the 2019 FDA public hearing on cannabis and cannabis-derived products, where it was concluded almost unanimously that we need a more rigid quality control of the industry.
While QR codes and Certificates of Analysis might bring a sense of comfort to a buyer, a closer look at the testing industry will reveal its elusiveness: there is great variability of lab results, lack of standardization and oversight, and sometimes a mere unwillingness to implement the existing rules.
It all means that the consumers have to be proactive while purchasing in a still unregulated market.
It also means that you should know things beyond labels and certificates.
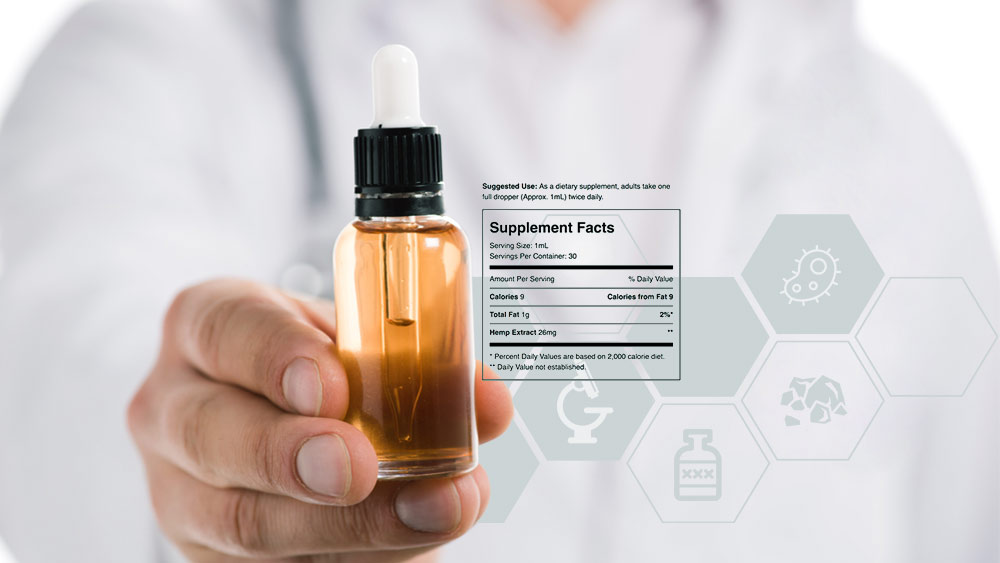
What Is a Certificate of Analysis on CBD Products, and What Should It Contain?
A Certificate of Analysis is (or should be) a quality assurance report, issued by an independent laboratory, that confirms a product meets its specification.
In plain words, it’s a result of lab testing that tells you what’s in the bottle. At the same time, this piece of paper is the single most important thing that should drive your purchasing decisions.
When it comes to CBD products, Certificates hold especially critical relevance.
Labels should provide information about the presence of potentially harmful contaminants as well as clear description of contents to allow consumers to confidently make decisions about potential therapeutic effects.
So, the first thing a CBD product should have listed on its Certificate is its cannabinoid profile. It should state the exact amount of each cannabinoid present and this is usually referred to as the potency of the product.
In addition, many trustworthy brands include a terpene profile, which offers insight into precise concentrations of the essential oils extracted from the plant.
This is, of course, only applicable to full spectrum and broad spectrum CBD products (isolates, by definition, only contain a single compound – usually THC or CBD).
But, when it comes to CBD products, sometimes it’s more important to note what’s NOT in the bottle. That’s why many brands choose to test their products for pesticides, heavy metals, microbes, and solvent residues.
To be concise, nobody is probably adding dangerous chemicals to their products. However, due to manufacturing practices, it is possible that some of them may end up in the finished preparation.
A full-panel certificate will address this issue and assure you that harmful compounds were non-detected.
How Reliable Are CBD Certificates of Analysis?
The trouble is that Certificates are not always what they should be.
A recent study by a team of scientists from the University of Mississippi showed how labels on CBD products can be misleading.
When we spoke to Dr. Mahmoud ElSohly, one of the team members who tested the samples, he recalled that a lot of the products had “legitimate” Certificates of Analysis.
But they often “had nothing in them,” he noted, which means that the Certificates were deceptive and misleading.
He’s not alone in his skepticism. Jaclyn Bowen, from the Clean Label Project, expressed a concern that poor instrumentation sensitivity could sometimes be the reason the test does not detect an analyte.
“One of the issues that I routinely see are laboratory Certificates of Analysis that use poor levels of detection and poor levels of quantification that yield “ND” (non-detect) test result,” Bowen said.
She went on to explain that if a laboratory only tests down to parts per million, a contaminant that is only present in parts per billion would not be detected.
“To a possible untrained eye, an “ND” may give a false sense of comfort, security, and compliance to the brand, which is confiding in those test results in good faith,” she concluded.
Standards Behind the Scenes
The main trouble with the lab testing industry is that it still lacks standardization and strict implementation. The CBD market has exploded almost overnight, and it seems that regulations have trouble keeping up.
To find the reason for this discrepancy, we spoke to Dr. Susan Audino, a chemist and chemometrician, who has various roles within the testing industry: she is a consultant to cannabis laboratories, assessor/auditor to ISO/IEC standards (most specifically to ISO/IEC 17025).
Also, she has chaired the AOAC Cannabis Advisory Panel and Cannabis Working Group, and was recently appointed the Scientific Advisor for the Cannabis Analytical Science Program.
Perhaps more importantly for this article, the presentation she gave on the 2019 FDA public hearing explicitly stated that impartial testing should be behind “Every product. Every label. Every time.”
Being in the industry outside any corporate requirements, she has seen “tremendous variability in the test results of data provided by Certificates of Analysis, to a point where the CoAs themselves must be viewed with skepticism.”
But it is her impression that things are moving in the right direction.
“I have been working closely with AOAC International for the development of standard test methods, specifically for the cannabis-testing laboratories. Although several methods have been published, more are on the way,” Audino said.
She explained that state regulators have been tasked with an enormously challenging feat – to regulate something that they previously outlawed and prosecuted. In addition, many of the regulations do not appear to be based on empirical science, which is a problem for all involved.
“To my personal and professional delight, many of these regulators are reaching out to appropriate personnel and organizations. The AOAC International recently launched the Cannabis Analytical Science Program (CASP) to which regulators are constantly joining and participating,” Audino said.
“Through their participation, AOAC and scientists who have been developing and implementing standard test methods are able to provide education while simultaneously responding to their (stakeholder) needs in the development of standard test methods,” she added.
It is her hope that “in light of the FDA restraint from providing federal oversight, the states will continue their collaborative efforts to better address the global needs.”
ISO/IEC 17025
Dr. Audino’s presentation gave us all a startle by stating that “Laboratory Accreditation to ISO/IEC 17025 is not a guarantee.”
“I fully believe that all quality control testing labs should be ISO/IEC 17025 accredited. This is a standard that is in effect throughout the world by international and federal laboratories within and outside cannabis. It is the single most important standard to ensure both an effective quality management program, and the technical competence to perform the work.” she noted.
“However, many cannabis laboratories are new to the testing space and are being developed and facilitated by non-scientists or by scientists who are very young and likely not intimately involved with ISO 17025 requirements.” Audino added.
“Consequently, the standard is often seen as a set of documents that, once written, can be set on the bookshelf without thorough implementation.” she concluded.
Additionally, because there are so few standard test methods available, laboratories are required to develop and validate their own methods. Unfortunately, the breadth of this work is generally understated and not fulfilled to the highest degree of ‘science’.
Although well intentioned, the lack of fully validating test methods results in inadequate test methods, or test methods that include many ‘shortcuts’ that will supposedly improve laboratory efficiency to accommodate more customer samples.
“This model tends to work very well at first but eventually fails because data integrity drives the customer base, not the other way around,” she observed.
She was skeptical about the consumer’s ability to check if a particular Certificate of Analysis is telling the truth.
“Long way of saying that even if a consumer wanted to check the CoA, bad data is bad data. We as an industry need to get a better handle on this. The most efficient way to get ahead of it is to establish standard test methods, including standard sampling methods. Even the best test methods are compromised by bad samples.” Audino concluded.
Federal Oversight
As with many stories from the cannabis industry, this one also points to the need for urgent federal oversight.
“The FDA has been addressing false advertising claims made on various cannabis products. However, beyond this, there is no federal oversight of what ‘should’ be included on the label, or how ingredients can or ‘should’ be advertised.” said Audino.
“With or without federal oversight, standards will be helpful to some extent. However, standards about how to label products will not address the data in the label claims,” she added.
Although organizations are diligently developing standard test methods, which meet essential needs of cannabis consumers, it would be helpful for some federal oversight consistent with the rest of the food and feed industry, concluded Audino.
How Can You Protect Yourself?
The somewhat scary conclusion of our research is that there is no way of knowing if a CBD Certificate is telling the truth.
It doesn’t mean that fraud is everywhere: a lot of legitimate companies want to make their brands trustworthy and play by the rules. You just have to open your eyes widely and recognize them.
Naturally, there is no official guidance on how to do this, but our SlideShare presentation might come in handy. In short, you should always thoroughly research both the company and the piece of paper that claims proper testing was performed.
Look for the elements we marked in the presentation as important. If they are not there, consider it a red flag and move on.
Towards a Conclusion: “Good Science Takes Time.”
Dr. Audino emphasized how important it is to accept that scientific quality demands effort and time.
“Developing standards or standard test methods must be scientifically sound, empirically based, and defensible. This can and does take time.”
In the meantime, remember to base your purchasing decisions on your own portion of empirically based research.




Brian February 3, 2020 at 5:28 am
Great write, tweeting